RESP PL
Respiratory Panel
EPIC Test Procedure Code: LAB2374
Submit only 1 of the following specimens:
1. Nasopharyngeal swabs in viral transport medium (M6) or UTM transport media. Bronchial wash or BAL or nasal washing, 1 mL minimum volume. The specimen must be received intact in a sealed, sterile container.
Follow current PSC procedures for registering patient and preparing test order.
Nasopharyngeal swab collection
1. Obtain M6 viral transport media or UTM transport media.
2. Collect swab using the following CDC (Center for Disease Control and Prevention) guidelines:
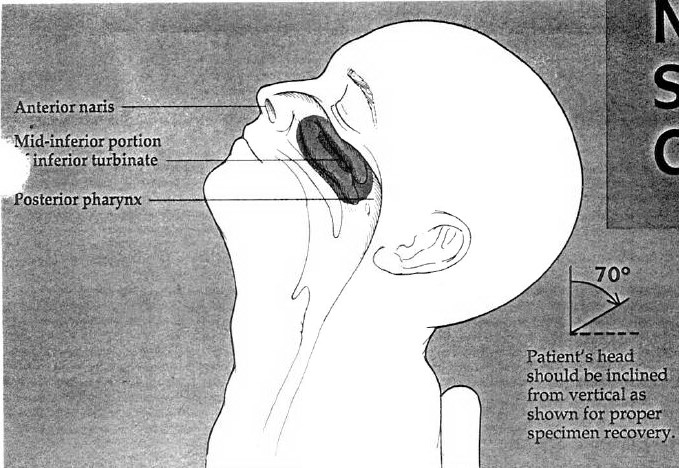
a. Tilt patient's head back 70 degrees.
b. Insert swab into nostril. (Swab should reach a depth equal to distance from nostrils to outer opening of the ear.)
c. Leave swab in place for several seconds to absorb secretions.
d. Slowly remove swab while rotating it. (Swab both nostrils with same swab.)
e. Place tip of swab into M6 viral transport media or UTM transport media or 0.5 mL to 1 mL of sterile saline and snap/cut off the applicator stick.
3. Label the tube with the patient’s full name, date of birth, identification number, date and time of collection, initials of the person collecting the specimen, and the specimen source.
4. Maintain sterility and forward promptly at room temperature only.
Note: The specimen source is required on the request form for processing.
Bronchial wash or BAL or nasal washing
1. Label the container with the patient’s full name, date of birth, identification number, date and time of collection, the initials or other unique identifier of the person collecting the specimen.
Note: The specimen source is required on the request form for processing.
Sputum, tissue, charcoal swabs or wooden swabs will be rejected.
0202U - Bill Resp PCR (NFCT DS 22 TRGT SARS-COV-2) 0202U (EAP 30047363)
* If detected, the isolate type will be specified.
Not detected